The use of dissimilar metals in the automotive industry has been a concern ever since its inception, when an automobile manufacturer attached a piece of aluminum hardware to a steel body only to see it corrode later.
Although I have a keen interest in metals, I’d like to state that I’m not a chemist, engineer, metallurgist or professor. I’m a collision repairman who happens to own a collision repair shop and has a keen interest in most things technical. In high school, my two favorite subjects were geometry and chemistry. As it turns out, both those subjects play a prominent role in collision repair.
Whether those same subjects ever piqued your own interest or not, I think you’ll agree with me that if we understand how and why something works or was designed to work, we’ll have greater success when it comes to repairing it. And I think you’ll find that this general philosophy applies to most automobile repair situations. If we just take a few minutes to diagnose a situation and understand why something is the way it is, we’ll reduce our exposure to failure and make those few minutes of diagnostic consideration well worth it.
So keep in mind that the comments, observations and remarks that follow are from a man with 44 years of practical, daily experience and enough curiosity to research and delve into the technical aspects of my observations. This is what can happen once the hormones ebb and sex, or the thought of sex, is no longer consuming a major portion of your mental process and alcohol is also just distant memory (29 years last December). The mind is free to delve into the big questions in life…like galvanic corrosion.
What the Heck Is It?
Galvanic corrosion, sometimes mistakenly called electrolysis, is the result of the reaction between at least two dissimilar metals in electrical contact with one another, in the presence of an electrolyte. The manifestation of this is a white powdery corrosion that forms on the surface of an aluminum part where it contacts a steel part. This generally bubbles the paint and deteriorates the aluminum. Many other metals suffer galvanic corrosion, but in our industry, the primary metals affected by this malady are aluminum, zinc and magnesium.
A good example of galvanic corrosion is what happened in the 1970s, when the Ford Motor Company produced chrome steel bumpers with aluminum reinforcements bolted into them. Ford then bolted the assembly to the frame horns of its automobile, using stiff paper shims as insulators between the reinforcement and the frame brackets. You had the electrical contact between the outer face bar and the aluminum reinforcement. What’s more, after the first wreck, collision repairers, myself included, looked at those stiff paper insulators and thought, “These guys are so cheap, they can’t even give us real metal shims!” and then threw them in the dumpster because the bumper fit amazingly well without any paper shims. They would just get soggy anyway, we thought. But by throwing those paper shims away, we created electrical contact between the frame and the aluminum reinforcement.
So…what is an electrical contact? It’s when two dissimilar metals make physical contact with one another (tight), allowing ions to travel between the metals. To make them reactive with one another, you need an electrolyte.
Where do you get an electrolyte? Well, you don’t need to go get it. Mother Nature supplies it in the form of rain and road splash. Man improves on it a little with the addition of de-icers or road salt, which provides an even better conductor for those ions to travel in.
So, the end result of the Ford situation was that we created a galvanic corrosion cell, or…a battery! Right out there on the front of your road warrior, you were using a battery for a bumper. Wow!
Were the OEs really that dumb? To be honest, in this scenario, we repairers helped them along the path to corrosion significantly by removing those paper insulators. But then it turns out that different metals react differently to aluminum in regard to galvanic corrosion. The chrome-plated bumper and the cadmium-plated bumper bolts didn’t react significantly to the aluminum bumper reinforcement – unlike the way the frame of the car (without those paper shims) did, which was basically raw, bare steel and unplated bolts. None of us had a clue! I saw the aftermath of that “battery for a bumper”
situation several times in subsequent years and thought, “Boy, those morons better rethink the
use of that soft aluminum for a bumper reinforcement.”
Now that I have more experience and understand things a little more, that series 7000 aluminum bumper reinforcement was plenty strong if we would have just put those little paper insulators back between the reinforcement and the frame and not allowed the effects of galvanic corrosion to compromise the more than ample strength it had. It would have worked as designed, and this goes right back to taking the time to understand why things are the way they are.
Metals React Differently
Figure 2 shows a condensed arrangement of metals in galvanic series. As you’ll observe, the metals on the lower end are called “protected.” These are the cathodes of a galvanic corrosion cell or battery. The metals on the very end, gold and platinum, are considered “most noble.” Platinum and gold don’t corrode. This is why, in the interest of safety, the components of sensitive aircraft and space travel instruments are made of gold and platinum plating.
|
Figure 2. Arrangement of Metals in Galvanic Series
|
|
|
CORRODED END
|
Any one of these metals and alloys will theoretically corrode while offering protection to any other that’s lowerin the series, so long as both are electrically connected.
In actual practice, however, sizn is by far the most effective in this respect. |
|
Anodic or less noble
|
|
|
Magnesium
|
|
|
Zinc
|
|
|
Aluminum
|
|
|
Cadmium
|
|
|
Steel
|
|
|
Lead
|
|
|
Tin
|
|
|
Nickel
|
|
|
Brass
|
|
|
Bronzes
|
|
|
Copper
|
|
|
Nickel-Copper Alloys
|
|
|
Stainless Steels (passive)
|
|
|
Silver
|
|
|
Gold
|
|
|
Platinum
|
|
|
PROTECTED END
|
|
|
Cathodic or most noble
|
|
The top end is called the corroded end. This is the anode on our battery, and the metals on this end of the series are “less noble.” The closer one of these metals is to another in the series, the less reactive it will be. The farther apart, the more reactive. For example, when steel is fastened to aluminum in the presence of an electrolyte, the aluminum is anodic to steel and will corrode much faster than it would by itself in the electrolyte. However, the steel, which is cathodic to aluminum, will corrode much slower than it would by itself in the electrolyte. Interestingly, the aluminum is protecting the steel.
This is why zinc, with its anodic, protective and sacrificial qualities when applied to steel and steel fasteners, is used extensively for coatings over steel.
Hey, maybe you’re not working on aluminum intensive vehicles, so you figure, “What’s this got to do with me?” But there are a few vehicles out there, F-150s, 250s and 350s, along with Buicks, Suburbans, Escalades and Toyotas, etc., that have some bolt-on hoods, hatches, deck lids, fenders, shields, etc. made from aluminum, and all are susceptible to galvanic corrosion if improperly treated. So get used to seeing aluminum because we’re going to see a lot more of it. The more aluminum that gets into the market, the more economically feasible it’s going to be to use it, because it costs a small fraction to recycle it than it did to smelt it in its original form.
Avoid Adverse Ratios
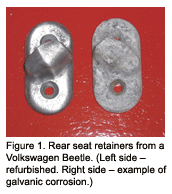
You need to avoid a large cathodic ratio compared to a small anodic ratio. What am I talking about? An example of this is the rear seatback retainer in an old Volkswagen Beetle. These were roughly two square inches of cast aluminum bolted to the steel car. A big adverse ratio and galvanic corrosion went wild. You’ll find a fuzzy looking pitted chunk of aluminum on the rear quarter sidewall of most VW Beetles (see figure 1).
Here’s how this can manifest itself on a modern car. I removed the hood from a Ford Escape recently and noticed that the area under the hood hinge was unpainted, but everywhere else on the underside of the hood was painted. The area where the steel hood hinge (painted everywhere) bolted to the aluminum hood was bare aluminum. By painting only the cathodic steel hinge, the manufacturers are trying to prevent the adverse ratio. That way, the anodic area is larger than where the cathodic steel hinge might have a mounting scratch on its contact surface. If the OE painted the mounting area of the hinge and that coating was nicked in the mounting process, a large adverse ratio
would be present in the small scratched area, causing vigorous galvanic corrosion.
 When bolting dissimilar metals together or even similar aluminum metals to an aluminum body, always use cathodic bolts – never anodic. In other words, you’ll use steel bolts. These bolts will most likely be coated with Dacromet (see figure 3), which is a special coating applied to steel bolts by different hardware manufacturers under license from Dacromet. The coatings should always be inspected to detect compromised protective coating upon removing a bolt. These coatings cannot be repaired and the fastener, which is coated and insulated and recommended by OEs to be replaced every time one is removed, must be replaced. When fitting parts with these bolts, use the original bolts for fit-up and, when you’re done, replace them with new bolts. Be aware. If the factory is going to this additional effort and expense, you should know it’s for a serious reason.
When bolting dissimilar metals together or even similar aluminum metals to an aluminum body, always use cathodic bolts – never anodic. In other words, you’ll use steel bolts. These bolts will most likely be coated with Dacromet (see figure 3), which is a special coating applied to steel bolts by different hardware manufacturers under license from Dacromet. The coatings should always be inspected to detect compromised protective coating upon removing a bolt. These coatings cannot be repaired and the fastener, which is coated and insulated and recommended by OEs to be replaced every time one is removed, must be replaced. When fitting parts with these bolts, use the original bolts for fit-up and, when you’re done, replace them with new bolts. Be aware. If the factory is going to this additional effort and expense, you should know it’s for a serious reason.
Cross Contamination
Beware of spreading aluminum sanding and grinding dust onto steel cars or parts and vice versa. This is easier than you think. All you need to do is pick up a sander that you just used on a steel car and use it with the same piece of sand paper on aluminum. This is cross contamination, and galvanic corrosion can be the result. The most likely result is bubbles in the finish. Take special care when working around bare aluminum. Most OE-certification programs require separate rooms for aluminum work. A different set of tools may also be required. Wipe power sanders down with a damp cloth before beginning. Don’t blow tools or sanding dust around aluminum vehicles or cars with aluminum parts on them because it floats in the air. Instead, use a vacuum. A little common sense and caution can prevent problems that might be costly to fix later.
Aluminum Oxide and Anodizing
Aluminum oxide is a naturally occurring oxidation that forms on bare aluminum almost instantly. It protects the aluminum surface from corroding further in most cases. Sanding or wire brushing (with a stainless steel brush) will remove it. It needs to be removed before priming or welding. It’s the second hardest material in the world after diamond, and its melting temperature is more than 3,700 degrees Fahrenheit. Aluminum melts at about 1,200 degrees Fahrenheit, so this causes problems when welding, if it’s at the weld site. Remove it with a stainless steel wire brush immediately before welding and sand the bare aluminum just prior to painting.
And beware: Aluminum oxide is
usually invisible.
Anodizing is a process used to protect aluminum from corrosion and is actually a manmade deposition of aluminum oxide. It’s much thicker than naturally-occurring aluminum oxide, is used as a decorative coating on moldings and trim, and can act as an electrical insulator.
Antifreeze as Electrolyte
Sure…think about it. You’ve got a pressurized, closed loop cooling system in a modern automobile.
There are dissimilar metals within the engine/cooling system. Let’s see…we’ve got a cast iron engine block with an aluminum radiator. They’re not in contact with one another but they’re in a pressurized closed loop system full of electrolyte. Oh, that electrolyte (antifreeze) that we’ve all taken for granted for so long. Each OEM has specific antifreeze that it uses to inhibit corrosion. When you work on a cooling system and remove the antifreeze, have the dealer send you its replacement antifreeze so you not only protect the cooling system but also the vehicle owner warranties and yourself.
|
The Lasagna Effect
This is a well-known scenario that has had wives and hungry husbands scratching their heads and Well, you’re right. The wife created a battery with the steel pan and aluminum foil, and the salty lasagna was the electrolyte where it made contact with the covering foil. |
Writer Mike West is a former contributing editor to BodyShop Business and was a shop owner for more than 30 years and a technician for more than 40 years before closing his Seattle, Wash., shop and retiring.